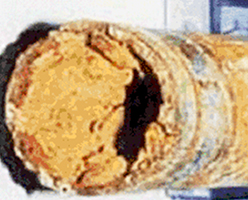
Corrosion and Cathodic Protection System
HOME > Introduction to Cathodic protection > Corrosion and Cathodic Protection
Causes of corrosion
Every metal possesses its own potential based on the surrounding environment (soil and underwater). This potential is influenced partially by the material's internal factors such as composition, stress, scale, and the environmental non-uniformities like resistance, temperature, humidity, and oxygen concentration. The partial potential difference arising from these factors leads to the flow of current, and the points where current is discharged are prone to corrosion.
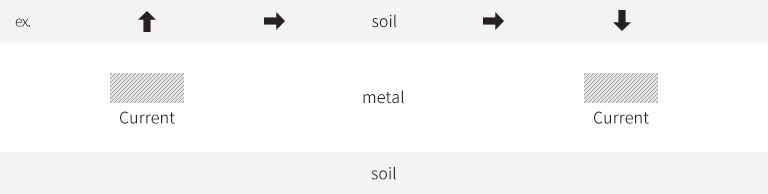
Low potential
Anode
Discharge current through soil
High potential
Cathode
The discharged current flow in
Corrosion condition
The corrosion of metal is influenced by various environmental factors, and the common three conditions for corrosion are existence of conductor (metallic connection), the presence of electrolytes (Soil, water), and the presence of a potential difference.
- Existence of conductor
(metallic connection) - Presence of electrolyte
(soil, water) - presence of potential difference
(Anode, Cathode)
Corrosion prevention methods
The methods for corrosion prevention involve blocking the three conditions for corrosion, which include coating, insulation, and cathodic protection.
| Three condition for corrosion | Prevention method | Anti-corrosion of metal |
|---|---|---|
|
Existence of conductor |
insulation: disconnection of metallic connection |
|
|
Presence of electrolyte |
Coating : block contact with electrolyte |
|
|
presence of potential difference |
Cathodic protection : eliminate potential difference |